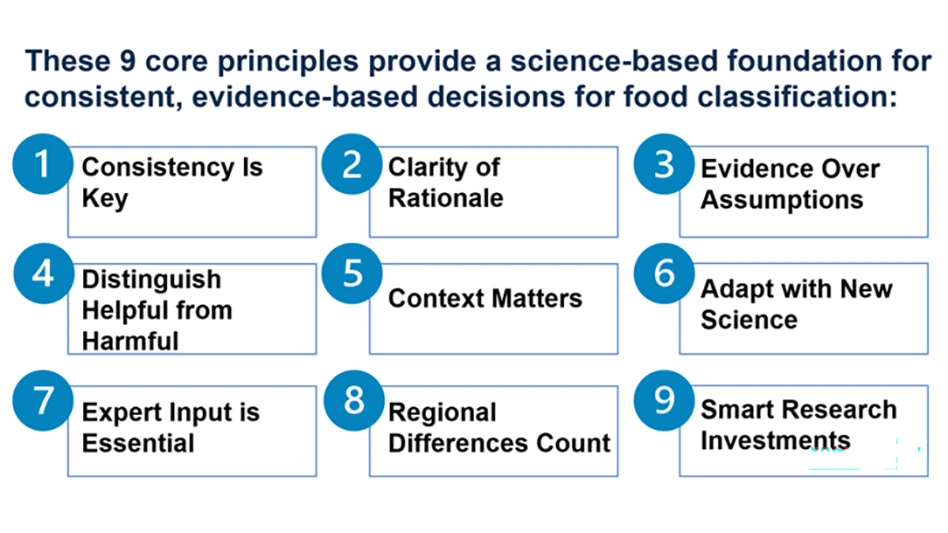
The U.S. Food and Drug Administration (FDA) released its Expanded Decision Tree (EDT) chemical toxicity and risk screening tool July 30.
The tool was designed to provide a consistent, systematic, science-based approach to support evaluation of the safety of chemicals in food based on their structure and estimated toxicity, said the agency. The EDT was evaluated through external peer-review, and FDA said it will engage stakeholders and the public for further feedback on the tool.
The EDT is an example of the FDA’s New Approach Methods, which leverage large data sets to achieve faster, less expensive, informative new approaches to chemical assessments that can inform risk management decisions and actions, according to the agency. The EDT has gained widespread international support in recent years, said FDA.
This approach to evaluating the safety of chemicals is a modernized version of the original Cramer Decision Tree tool and can be used to screen chemicals based on their structural features. The Cramer Decision Tree tool sorts chemicals into classes of chronic toxic potential using a series of mainly chemical structure-based questions and has been widely used by scientists to provide a quick, preliminary estimate of a new chemical’s predicted toxicity, especially when the testing data about a chemical is limited, said FDA.
The updated, expanded and refined set of fully chemical structure-based questions in the FDA’s EDT allows classification of chemicals with greater specificity than the Cramer Decision Tree, said the agency.
The EDT will help inform the nature and extent of additional testing or evaluation that may be needed to help address potential data gaps for chemicals in food, said FDA. The tool is expected to eventually be used in both pre- and post-market evaluation of chemicals in food to help ensure the food supply remains safe, according to the agency. The FDA said it anticipates that the EDT will provide information that can be incorporated into the agency’s prioritization of chemicals for post-market review.
In March 2024, the FDA submitted the EDT for external peer review. This review collected input from external scientific experts in line with the requirements of the Information Quality Act. The FDA updated the EDT based on the review and is now publicly releasing it to the scientific community for technical consideration.
The FDA is developing a software solution for the EDT for general public use and will further refine the EDT over time with access to more information about chemicals in the food supply and based on public input, according to the agency.
The FDA said it will release an informational video to explain the EDT and will hold listening sessions to receive input from interested parties. More details will follow.
Latest from Quality Assurance & Food Safety
- New Strain, More Illnesses Reported in Moringa Leaf Powder Salmonella Outbreak
- Kiwa ASI Expands Canadian Presence with Addition of TSLC
- Samples Collected by FDA Test Positive for C. Botulinum in ByHeart Infant Formula Investigation
- FDA Releases Total Diet Study Interface
- Wisconsin Cheese Makers Association Offers Student Scholarships
- Mandy Sedlak of Ecolab on Building a Career in Food Safety and What’s Next for the Industry
- Kroger Shares 2026 Food Trend Predictions
- USDA Announces New World Screwworm Grand Challenge